CASE STUDIES
Mack Molding
The selection of Mack Molding Case Studies shown below represents only a few examples of the Company’s medical manufacturing services and how they have helped customers resolve challenges and move product to market. It is not intended to provide a comprehensive listing of Mack’s Molding’s involvement in any industry or all the information on medical case studies available.
Mack Molding Medical Case Studies: Case & Tray Delivery System
 As the knee system was being developed, the customer wanted to design an instrumentation set, including related cases and trays, that would accent the revolutionary implant. At that same time, the customer was already working with Mack Molding to co-develop ergonomic overmolded handles for their top-of-the-line titanium instruments for the system. During a tour of its headquarters plant, they noticed Mack Molding’s significant sheet metal fabricating capabilities in addition to its molding expertise and how they both could be leveraged.
As the knee system was being developed, the customer wanted to design an instrumentation set, including related cases and trays, that would accent the revolutionary implant. At that same time, the customer was already working with Mack Molding to co-develop ergonomic overmolded handles for their top-of-the-line titanium instruments for the system. During a tour of its headquarters plant, they noticed Mack Molding’s significant sheet metal fabricating capabilities in addition to its molding expertise and how they both could be leveraged.
The Challenge
Getting there wasn’t easy, however, as the system needed to:
- Properly package the new flagship product by reflecting the same branding initiative, so the system would be immediately recognizable by OR staff
- Consolidate the number of instruments and cases to use space more efficiently in the surgical theatre and minimize costly cleaning procedures
- Configure the instruments in stackable modules that correspond to surgical procedure and flow
- Withstand shipping and handling
- Remain under a specified weight for ease of handling
- Resist harsh chemicals used to meet worldwide cleaning standards
- Provide adequate steam flow during autoclaving to thoroughly clean instruments and eliminate wet-packing
- Provide instrument constraint with minimum contact for improved cleaning and autoclaving
- Stack in a sterility tub for shipping, which requires standardized sizes
- Avoid sharp edges that tear the sterile wraps and compromise the sterility of both the system and the surgical theatre.
“Time was another challenge,” says Chris Wartinger, Mack Molding Business Unit Director. “Cases & trays traditionally are the final piece of the puzzle. The design team has to finish the instruments and protocol first, which typically require some last minute tweaking to satisfy user needs. Those changes move down the line, impacting the delivery system as well. For example, if you make an instrument handle two millimeters larger, then we have to similarly adjust all brackets in all cases & trays, as well as all assembly prints. Consequently, we generally develop cases & trays when the rest of the program is 80 percent complete, leaving 100 percent of the work to do in 20 percent of the time.”
The Solution
The collaborative design team addressed the branding initiative with material, color and corporate identification. Titanium became the material of choice because it not only addresses several performance criteria, but also matches the instrumentation and distinguishes the sets from all others in the field. All plastic resin used on the instrument handles and case is custom formulated to match the customer’s brand. Both company and product identification are laser-cut into the case and molded into the instrument handles.
The redesigned instruments feature a basic handle style with ergonomic soft-grip handles that are insert molded with a double overmold. The first material is a high heat polypropylene, and the second is a custom blended, thermoplastic elastomer (TPE).
To address the consolidation issue, the handle design introduces a quick attach-and-release mechanism that saves time in fixture assembly and minimizes the number of instruments required.
Downsizing the number of instruments allowed for consolidation of cases & trays as well.

The case & tray sets are two-tiered systems configured intuitively to correspond to the surgical procedure. They are laser-cut from titanium, a lightweight but durable material that withstands harsh cleaning solvents, as well as the customer’s rigorous testing procedures.
Numerous aeration holes are laser-cut into the case to provide a sufficient flow of steam during autoclaving, as well as proper ventilation for drying. In addition, titanium brackets provide adequate instrument constraint with minimum contact for improved cleaning and autoclaving.
Mack Molding also uses laser welding and special forming techniques to support the instrumentation with titanium brackets. The titanium is anodized as well, for an anti-galling effect and improved silk screening and cosmetics.
The corners of the case are fitted with plastic bumpers molded of TPE for shock absorption and sterilization protection, since the TPE eliminates rough edges and corners. Plastic handles are also integrated into the trays. Again, both are color matched to the branding initiative, making the delivery system easily recognizable as the customer’s product, both in the OR and the marketplace.
Resources Required
To better accommodate the customer’s case & tray program, Mack Molding invested $1.4 million to shorten the supply chain and lengthen its vertical integration in sheet metal fabrication, a core competency. The Company added more laser-cutting capacity, as well as laser welding and other secondary operations, meaning the product is manufactured almost completely in-house, helping minimize risk in the supply chain.
For more information on these or other Mack Molding medical case studies, contact salesnorth@mack.com.
……………………………………………………………………..
Mack Molding Medical Case Studies: Inventory Management System Medical Case Study

So how do these RFID cabinets help people make better decisions? The real dollar savings come from the data that allows staff to answer critical bottom-line questions:
- What IS the best deal I can get for this lab?
- Should I buy in bulk?
- Should I consign products?
- Should I reduce the variation of vendors?
At a time when cost reduction in health care could not be more critical, data is crucial. Especially when 10-15 percent of the volume of products in a hospital represents 80-90% of the cost. Of the some 15,000 items on a hospital’s regular shopping list, about 85 percent cost $5-10 apiece. Syringes, drapes, bandages, products they buy a lot of. That leaves 2500 or so items that average around $800 each. So there’s a steep cost differentiation between the bundle of cheaper products and the expensive medical devices, where they need just enough, but not too much due to the cost burden.
How it works
That’s where this clinical inventory management system, comes in. RFID tags are placed on each item as it’s received through the hospital supply chain. Product information is captured from the product bar code, assigned to the RFID tag, and saved into the system’s database for real-time medical device tracking via the internet. No huge IT investment that has to be embedded into the hospital data center.

Hospital staffs simply wave the item at the Point of Service reader to capture its usage, resulting in more accurate target inventory, product replenishment, and charge capture. Users logon to the internet and view a host of user-friendly reports and analytical tools to effectively manage inventory supply levels. A customer-driven company, the customer focuses a lot of energy on customizing the reports to the individual needs of hospitals, so there are generally three or four new web-based software releases annually. But they are all included in the subscription. No more expensive upgrades.
And yes, it is a subscription service, not a capital acquisition. The customer retains ownership of the equipment. Hospitals and surgi-centers subscribe to the service, which the customer updates continuously. No more technology obsolescence.
Medical OEMs, with lots of product in the field on consignment, are also benefiting. Historically, these manufacturers have struggled with a lack of visibility to these expensive resources with no way to track expired product. With this solution, they can provide better service to their customers, a more cost-effective supply chain for their shareholders and, ultimately, improved patient safety.
Mack Molding’s involvement
The customer chose Mack Molding as its manufacturing arm. There are several RFID-enabled cabinets in the customer’s portfolio, including a five-shelf cabinet, five-shelf cabinet with locking doors, hanging cabinet with six hangers, bin for tall boxes, mobile four-shelf cabinet, tabletop one-shelf cabinet, and a hanging cabinet with doors that is still in development.
All models feature RFID technology and are built of high grade steel and engineering thermoplastics. They are each subjected to a rigorous cycle of quality testing that includes:
54-step build check sheet to verify proper configuration of critical element
- Hi-Pot test to ensure power supplies and safety grounds are correctly configured
- Several software checks for proper configuration and setup
- Physical checks on motors
- Communication/connectivity checks between boards, computers, motors
- Full performance test with calibrated test set to simulate actual use
- 48-hour burn-in with second full performance test
- Verification of communications within cabinet and from cabinet to server
- Final quality audit prior to packaging.
Additionally, all work instructions are formatted with quality checks at key points of subassembly.
For more information on these or other Mack Molding medical case studies, contact salesnorth@mack.com.
……………………………………………………………………..
Mack Molding Medical Case Studies: Defibrillator Handle Medical Case Study
CAE teams with gas-assist for defibrillator solution
Another of Mack Molding’s medical case studies involves 911 caller complains of chest pain, shortness of breath. EMTs speed to the scene, ambulance defibrillator in tow. When they arrive, assessing and treating the patient is Priority #1. Time is precious and the equipment must perform.
Designed to meet the specific demands and extreme conditions of the emergency medical services environment, this ambulance defibrillator features a suitcase-style design with an innovative protective roll cage, allowing emergency personnel to carry and store the device easily. Lightweight, yet indestructible, it can be dropped off a building or run over by a truck without affecting operation.
The unit is protected on three sides with gas-assisted parts – two side rails and the front handle, the subject of this case study. The handle is used both to carry the unit and set it up at an angle. Therefore, it has to be durable and sturdy enough to carry the 15-lb device, yet comfortable and easy to grip for the emergency medical technician handling the product. (Image on left: A cross-section of the finished over-molded handle clearly illustrates the gas channel, polycarbonate substrate and TPU overmold.
The Challenge
A key element of the product’s exterior design is the light, hollow injection molded handle. The customer wanted a handle that was hollow, but stiff enough to pass rigorous tests. Specifications also included a soft-touch feel for easier transport and a snug grip. There could be no gas pinhole in the handle, and both the substrate and overmolding resins had to be capable of developing a chemical bond.
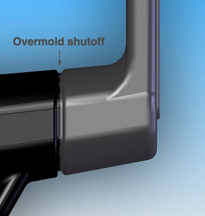 While hollow, the substrate also had to be structural enough to withstand a shutoff in the steel tool to prevent flash. To accomplish this, the tooled steel had to apply just the right amount of pressure to the substrate to stop the flow of the overmolding resin without crushing the substrate. (Figure on right: The shutoff that defines the boundary between the overmold (black) and substrate results in a grooved design.)
While hollow, the substrate also had to be structural enough to withstand a shutoff in the steel tool to prevent flash. To accomplish this, the tooled steel had to apply just the right amount of pressure to the substrate to stop the flow of the overmolding resin without crushing the substrate. (Figure on right: The shutoff that defines the boundary between the overmold (black) and substrate results in a grooved design.)
CAE Confirms Solution
To produce a handle that will pass all the requirements, the first step is to choose the right gas-assist process.
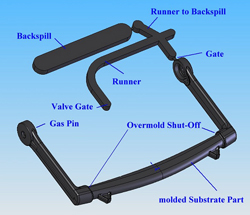 In this case, the best technique is full-shot molding with backspill, which results in a complete filling of the cavity with melt, followed by gas injection and opening of the backspill cavities. (Figure on left: Molding concept for gas-assist handle for automated external defibrillator (AED).
In this case, the best technique is full-shot molding with backspill, which results in a complete filling of the cavity with melt, followed by gas injection and opening of the backspill cavities. (Figure on left: Molding concept for gas-assist handle for automated external defibrillator (AED).
Mack Molding used CAE and various flow simulation software to determine the size of the backspill cavity, discreetly position the gas pin, and avoid a hole at the gating location. Flow simulations also clearly illustrated the remaining wall thickness distribution of the substrate part after we introduced gas into the process. (Figure on right: Flow simulation traced the path of the gas channel and illustrated wall thickness distribution.)
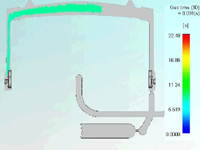
The handle is molded in a 300-ton press because of tool size, which incorporates the backspill cavity, valve gate, gas pin, four tool actions and a hydraulic shutoff between the runner and backspill cavity. The substrate is molded of modified polycarbonate resin for chemical resistance, flame retardancy and ECO compliance. It is overmolded with thermoplastic polyurethane for abrasion, required shore hardness, impact resistance, flow length and a comfortable grip.
Mack Molding molds a total of 14 parts for this ambulance defibrillator unit, using thermoplastic polyurethane, polycarbonate and modified ABS resins. All materials are high impact resins with high temperature resistance to assure performance in the harshest of conditions.
For more information on these or other Mack Molding medical case studies, contact salesnorth@mack.com.



 54-step build check sheet to verify proper configuration of critical element
54-step build check sheet to verify proper configuration of critical element










