MEDICAL
Mack Molding
Precision Manufacturing for the Medical Market
Your Partner in Medical Device Manufacturing
 With over 100 years of manufacturing expertise, including 25+ years in medical devices and orthopedics, Mack Molding is a trusted partner for Medical Device Manufacturing including cases & trays, implant trials, instrumentation, and robotic surgical devices. Our capabilities extend to plastic skins, sheet metal chassis, and complete assembly solutions, ensuring seamless production of critical medical equipment.
With over 100 years of manufacturing expertise, including 25+ years in medical devices and orthopedics, Mack Molding is a trusted partner for Medical Device Manufacturing including cases & trays, implant trials, instrumentation, and robotic surgical devices. Our capabilities extend to plastic skins, sheet metal chassis, and complete assembly solutions, ensuring seamless production of critical medical equipment.
From diagnostic and surgical devices to laboratory equipment, Mack Molding provides fully integrated, end-to-end solutions, including plastic injection molding, precision machining, sheet metal fabrication and final assembly. Our expertise in complex geometries, metal-to-plastic conversion, and advanced material processing allows us to meet the most stringent quality and regulatory requirements in the medical industry for Medical Device Manufacturing.
Our Proven Process: From Design to Production
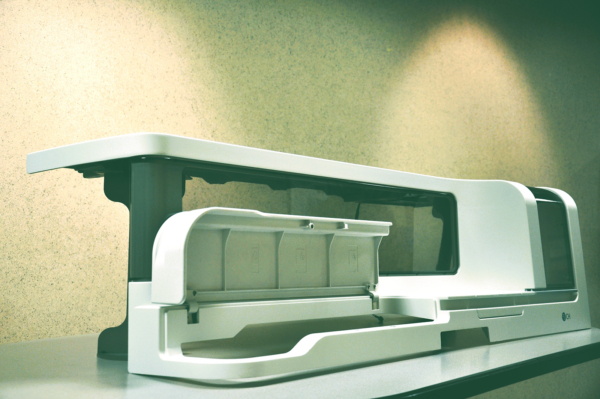 At Mack Molding, we partner with medical OEMs early in the development process, applying Design for Manufacturability (DFM) expertise to ensure optimized production and cost efficiency.
At Mack Molding, we partner with medical OEMs early in the development process, applying Design for Manufacturability (DFM) expertise to ensure optimized production and cost efficiency.
- Tooling & Process Development – Our in-house teams manage tooling, including design, tool build, and rigorous process validation.
- Regulatory Compliance & Validation – We are quality experts who understand OEM validation protocols and operate within large company Approved Vendor Lists (AVLs) with expertise in global supply chain management.
- Seamless Transition from Prototype to Full-Scale Production – Our vertically integrated approach ensures efficiency, quality, and speed to market from design and development through prototyping to full-scale production.
Comprehensive Manufacturing & Capabilities
Mack Molding specializes in precision injection molding and metal fabrication for a wide range of medical applications, including:
- Molded Skins for Diagnostic, Surgical, and Laboratory Equipment – Durable, ergonomic, and designed for seamless integration.
- Sheet Metal Chassis & Enclosures – Supporting structural integrity for high-performance medical devices.
- Cases & Trays for Surgical & Sterile Processing – Custom-engineered for durability and cleanability in hospital environments. Our cases and trays are engineered for superior sterilization and durability, offering key advantages:
- Laser Welding for Seamless Design – Eliminates hard-to-clean crevices, reducing bioburden accumulation.
- Metal vs. Plastic – Metal cases dry faster post-sterilization, reducing bacterial growth risks compared to plastic alternatives.
- Implant Trials & Instrumentation – Manufactured to precise tolerances for surgical accuracy and repeatability.
- Assembly of Robotic Surgery Devices – Supporting cutting-edge minimally invasive surgical systems.
 Value-Added Capabilities for Medical Manufacturing
Value-Added Capabilities for Medical Manufacturing
At Mack Molding, we go beyond standard manufacturing to deliver precision, quality, and compliance for medical devices and components. Our value-added capabilities ensure seamless integration, enhanced functionality, and superior reliability in every product we manufacture. We provide comprehensive solutions that meet the strictest industry standards, while helping medical OEMs streamline production for cutting-edge medical technology.
- Precision Machining & Sheet Metal Fabrication – In-house capabilities for machining, welding, and assembly.
- Overmolding – Creating soft-touch, ergonomic handles and implant trials branded to customer specifications using overmolding techniques.
- Laser Marking – Permanent markings for QR codes, UDI, CE marks, GTIN numbers, 2D barcodes, and branding.
- Metrology & Quality Assurance – Tight tolerances of 50 microns for machined parts and 80-100 microns for plastics.
- Metal-to-Plastic Conversion – Optimizing cutting guides, augments, trials, and sizing templates while retaining tight tolerances.
- FDA Registered & ISO Certified – Meeting ISO 9001 and ISO 13485 standards, with capabilities for Class III PMA medical device manufacturing.
- Class 100,000 Clean Rooms – Ensuring a controlled environment for critical medical components in both our molding and assembly departments
Why Choose Mack Molding for Medical Manufacturing?
 With decades of experience, cutting-edge capabilities, and unwavering commitment to quality, Mack Molding is the premier choice for precision medical manufacturing.
With decades of experience, cutting-edge capabilities, and unwavering commitment to quality, Mack Molding is the premier choice for precision medical manufacturing.
- Decades of Experience – Over 100 years of manufacturing expertise, including 25+ years in medical devices.
- Vertically Integrated Excellence – Complete molding, machining, assembly, and packaging solutions under one roof.
- Unmatched Quality & Compliance – ISO-certified, FDA-registered, and Class III PMA-ready.
- Engineering & Innovation – Collaborative design, prototyping, and material expertise for next-generation medical devices.
Case Studies
Read about Mack Molding medical case studies.
Let’s Build the Future of Medical Technology—Together. Contact us today to discuss your medical manufacturing needs.











